Abstract
Background. Allogeneic bone marrow transplant (BMT) is a potentially curative approach in patients with refractory or high risk hematologic malignancies, such as acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS). Prior to transplant, patients are prepared with non-specific, high dose chemotherapy alone or in combination with total body irradiation, which are associated with early and late morbidities, including organ toxicities, infertility, secondary malignancies, and substantial risk of mortality. As a result, many eligible patients do not consider transplant and of those transplanted, 2/3 can only tolerate reduced intensity conditioning, which is associated with increased relapse rates (Scott et al. Journal of Clinical Oncology 2017, 1154-1161). Thus, safer and more effective conditioning agents with improved disease control are urgently needed. To meet this need, we developed two novel antibody drug conjugates (ADCs) conjugated to amanitin (AM) targeting CD117 (C-KIT, Pearse 2018), which is expressed on hematopoietic stem and progenitor cells and AML and MDS cells in >60% of patients (Ludwig et al. Haematologica 1997, 617-621), and CD45 (Palchaudhuri 2018) which is expressed on all lympho-hematopoietic cells and nearly all hematologic malignancies except multiple myeloma. The aim of the project was to design an agent with the dual benefit of depleting primary human hematopoietic stem progenitor cells (HSPCs) while reducing disease burden in leukemia models.
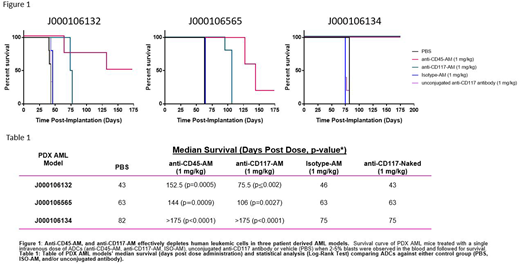
Methods. ADCs were tested in xenograft murine models inoculated with human AML cells from immortalized cell lines (Kasumi-1, a CD117 expressing leukemia cell line, and REH-Luc, a CD45 expressing AML cell line tagged with luciferase), and three patient-derived xenografts (PDX) developed from FLT-3+NPM1+ AML samples (J000106132 [prior treatment with Allogeneic BMT, Sorafenib, Hydroxyurea, and Decitabine], J000106565 [M4/5, prior treatment with Induction chemotherapy, Consolidation HiDAC, Allogeneic BMT], J000106134 [M4, no prior treatment reported]) with varying growth kinetics (median survival of vehicle treated groups was 43, 63, 82 days post inoculation) that express both CD117 and CD45 (Jackson Laboratories).
Results. In the Kasumi model, a single injection of 0.3 mg/kg anti-CD117-AM administered on day 7 or 42 after AML inoculation resulted in a marked increase in survival (median >240 days) compared to vehicle treated controls (median 76 days) or unconjugated anti-CD117 antibody (median 86.5 days) (n=6-8 mice/group, p<0.0001). In the REH-Luc model, a single injection of 1 mg/kg anti-CD45-AM on day 5 after AML inoculation resulted in longer survival by a median of 15 days compared to vehicle treated controls or unconjugated anti-CD45 antibody (n=10 mice/group, p<0.0001). For the three PDX, a single intravenous dose of ADCs (anti-CD117-AM, anti-CD45-AM, isotype-AM (ISO-AM), unconjugated anti-CD117 antibody, or vehicle PBS) were administered to AML-PDX animals when 2-5% blasts were observed in the blood. With 4-5 mice/group/AML-PDX model, survival was significantly increased in recipients of 1 mg/kg anti-CD117-AM, and 1 mg/kg anti-CD45-AM as compared to vehicle controls (Figure 1 and Table 1).
Conclusions. Anti-CD117-AM and anti-CD45-AM are potent anti-leukemia agents based on these data in humanized murine models with established AML. Together with prior reports on the potency of anti-CD117-AM and anti-CD45-AM as conditioning agents, these non-genotoxic ADCs may be useful to reduce disease burden in patients with active disease and in recipients of reduced dose conditioning who are at high risk of disease relapse.
Proctor:Magenta Therapeutics: Employment, Equity Ownership. Hyzy:Magenta Therapeutics: Employment, Equity Ownership. Adams:Magenta Therapeutics: Employment, Equity Ownership. Brooks:Magenta Therapeutics: Employment, Equity Ownership. Gabros:Magenta Therapeutics: Employment, Equity Ownership. McDonough:Magenta Therapeutics: Employment, Equity Ownership. Kien:Magenta Therapeutics: Employment, Equity Ownership. Aslanian:Magenta Therapeutics: Employment, Equity Ownership. Pearse:Magenta Therapeutics: Employment, Equity Ownership, Patents & Royalties. Palchaudhuri:Magenta Therapeutics: Employment, Equity Ownership, Patents & Royalties; Harvard University: Patents & Royalties. Li:Magenta Therapeutics: Employment, Equity Ownership. Kallen:Magenta Therapeutics: Employment, Equity Ownership. Sarma:Magenta Therapeutics: Employment, Equity Ownership. McShea:Magenta Therapeutics: Employment, Equity Ownership. Ladwig:Magenta Therapeutics: Employment, Equity Ownership. Dushime:Magenta Therapeutics: Employment, Equity Ownership. Panwar:Magenta Therapeutics: Employment, Equity Ownership, Patents & Royalties. McDonagh:Magenta Therapeutics: Employment, Equity Ownership, Patents & Royalties. Boitano:Magenta Therapeutics: Employment, Equity Ownership, Patents & Royalties. Cooke:Magenta Therapeutics: Employment, Equity Ownership, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal